BEAR Implant
What is Bridge Enhanced ACL Restoration (BEAR®)
A BEAR® implant is a new technology which acts as a bridge to help your body heal an ACL tear on its own, without a graft tendon from your body or a donor. A simple outpatient procedure, BEAR may facilitate faster recovery of muscle strength and has received high patient satisfaction for readiness to return to sports.
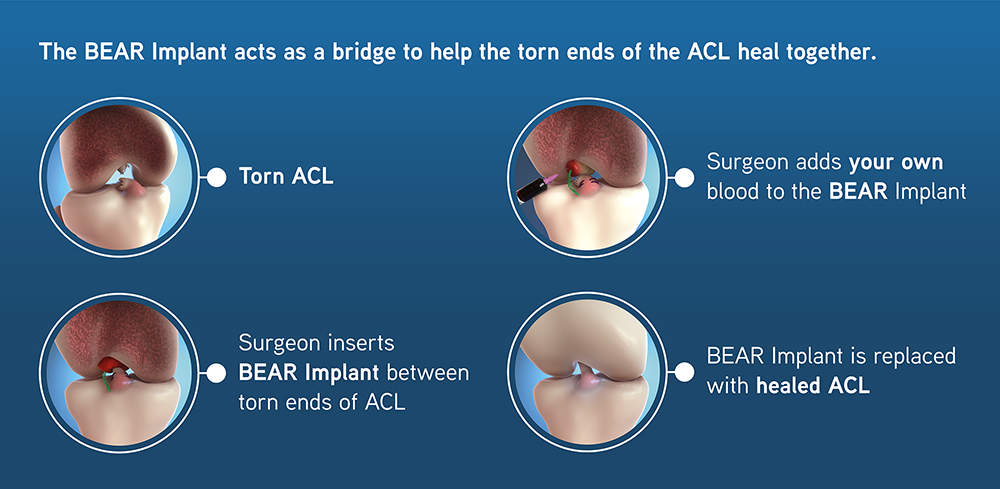
As seen above in images: The surgeon positions the BEAR Implant as a bridge between the torn ends of the ACL and sutures it in place. It is then injected with the patient’s own blood to facilitate healing.
The BEAR Implant facilitates the body’s healing response, supporting cell migration and proliferation.
Over time the BEAR Implant is absorbed and replaced with native cells, collagen and blood vessels, allowing the ACL to heal back together.

A torn Anterior Cruciate Ligament (ACL) in the knee is one of the most common injuries that unfortunately often plagues athletes. An ACL can’t heal itself and requires surgery, and often a tendon graft.
The BEAR technique combines suture repair of the (ACL) with a specific extracellular matrix scaffold (the BEAR implant) that is placed in the gap between the torn ends of the ACL, to facilitate and stimulate ligament healing. The scaffold acts like a bridge enabling the patient to heal their own tissue.
BEAR was developed and tested by Miach Orthopaedics and approved by the FDA in 2020. SOSI’s Dr. Meininger performed the first BEAR procedure in Central/Rocky Mountain States in December 2021.
What are the benefits of BEAR?
“The advantage of the BEAR procedure is that it avoids taking a tissue graft, so no extra incisions are required. None of the intact natural tissues need to be interrupted or harvested to perform this surgery.” –Dr. Meininger
BEAR does not require a graft tendon from the patient or a cadaver donor so there is no second wound site to heal, no worries about donor graft quality and no chance of tissue rejection.
Regenerating tissue is less invasive than reconstruction and puts less stress on the body.
For athletes and younger patients, there is an 86% return to sport success with BEAR surgery. There is a 70% return to sport with traditional ACL surgery.
Studies show that BEAR implants provide better hamstring strength than traditional ACL restoration at 6 months, 12 months and two years after the procedure.
Animal data suggests that the BEAR bioactive implant may reduce the risk of developing osteoarthritis after surgery. Human clinical studies are ongoing.
Are you a candidate for BEAR?
“One of the concerns with routine ACL reconstruction is the potential longterm outcomes; patients may still develop arthritis or damage to the joint because they had ACL surgery. The goal of BEAR and restoring the natural anatomy is that we allow the body to heal itself and ideally prevent some of these degenerative changes and over-tightening that can be associated with ACL reconstruction.” –Dr. Meininger
Athletes who want to return fully to their sport and younger patients who want to remain active throughout life are prime candidates for BEAR.
BEAR patients must be skeletally mature and at least 14 years old with a complete rupture of the ACL, and with the ACL stump still attached to the tibia.
BEAR must be performed within 50 days of the injury.
The recovery time for BEAR is similar to traditional ACL restoration with patients able to return to activity from 8 months.
BEAR at Steamboat Orthopaedic and Spine Institute
Dr. Alex Meininger, a founder and partner at SOSI, has trained in BEAR technique. In December 2021, he was the first orthopedic surgeon in the Central/Rocky Mountain states to successfully restore an ACL injury using BEAR and has since performed the surgery several times at the Steamboat Surgery Center. SOSI is a leading BEAR surgery location nationwide and patients have traveled from Reno, NV; Austin, TX; Santa Fe, NM; and Denver, CO to be treated by Dr. Meininger.
10th Bear implant - May 18th, 2022
Spinal Disc Replacement
- an alternative to spinal disc fusion
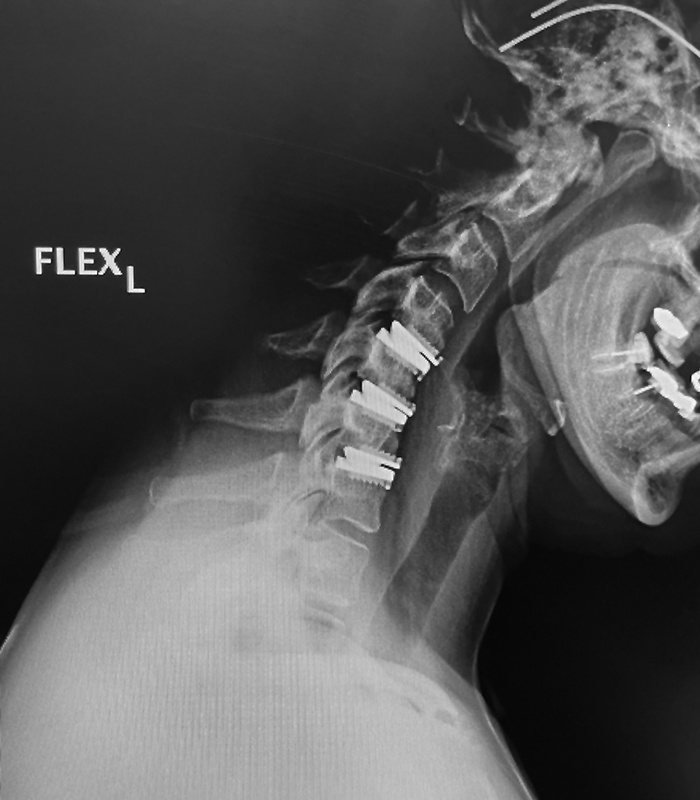
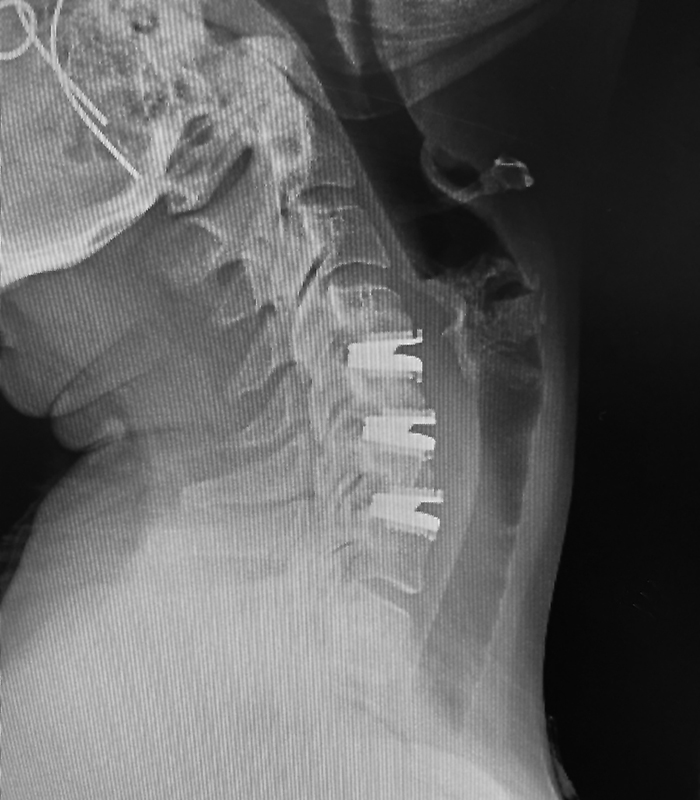
Preserving Spine Motion
Spinal disc replacements are increasingly becoming a preferred way to preserve spine motion and an alternative to fusion procedures which may lead to more rapid degeneration adjacent to the fusion, loss of flexibility and the need for possible future surgeries. Approved by the FDA in 2007, cervical disc replacements are now common, and Dr. J. Alex Sielatycki has performed over 400 of these procedures. SOSI is currently involved in trials toward FDA approval of more complicated lumbar disc replacements.
“The spine is not a rigid structure, by design, it is intended to move. Every time you move – sit, stand, bend, twist – it’s part of a kinetic chain that includes everything from the ground up, from your feet through your legs to your hips and pelvis to the spine.” - Dr. J. Alex Sielatycki
Dr. J. Alex Sielatycki, brings his strong interest and expertise in spine motion preservation to SOSI. The use of disc replacements throughout the spine provides an alternative to spinal disc fusion procedures, which may lead to more rapid degeneration adjacent to the fusion, loss of flexibility and the need for future surgeries.
Advancements in Lumbar Spine Replacements
Although disc replacements are common in the upper or cervical spine, due to the complex anatomy and the difficulty in working around nerves, joint replacements are not yet as common in the lower spine.
Currently Dr. Sielatycki, SOSI and the Steamboat Surgery Center are involved in a clinical trial performing the new BalancedBack Total Joint Replacement surgery of the lumbar spine segment using the breakthrough 3Spine MOTUS lumbar device, as is an alternative to spinal fusion surgery. This clinical series is moving the new device toward FDA approval. By maintaining or restoring motion of the lumbar discs, pain is relieved and collateral damage to adjacent segments is minimized.
“We have known for decades that lumbar spinal fusion surgery can have substantial negative downstream effects on spinal motion and overall spinal health, but truly effective alternatives to fusion have been elusive. With this lumbar total joint replacement technology, I believe we have an answer to this decades-long problem which has plagued so many patients. “ - Dr. J. Alex Sielatycki
Dr. Sielatycki is currently enrolling patients in the FDA clinical trial for the 3Spine BalancedBack Total Lumbar Joint Replacement, as well as into the standard of care “control” arm of the study.
